Our Studies
We currently have over 40 research studies in set up, recruitment or follow up. Our research portfolio is diverse and ranges from supporting complex single centre studies to national multicentre randomised controlled trials.
We receive funding from a variety of NIHR, Research Council and charitable funding streams. In total, our research studies cover over 400 sites and include more than 28,000 research participants.
Although we take on studies in a wide range of therapeutic areas, our main research fields are: cardiovascular, diabetes, dementia, musculoskeletal, psychology, respiratory and stroke. Within these we are investigating medical interventions, screening, rehabilitation, lifestyle modifications and health service delivery.
We have experience in designing and running a wide variety of studies, including trials of complex interventions, clinical investigations of medical devices, CTIMPs, behavioural interventions and trials utilising routine data. We have experience undertaking RCTs in both Primary and Secondary care and within the Care Home sector. We also undertake methodological research.
We are unable to support studies without funding. Funded single centre, and small feasibility trials may be considered for adoption by the CTU if there is a clear strategy of development to deliver a definitive phase III trial.
Please contact us for further information.
Discover more about some of our current research projects below.
Discover our ongoing studies:
A Proof of Concept Study for Vitamin A Nasal Drops in Post-Viral Olfactory Loss
Click here to learn more
At-Risk Registers Integrated into primary care to Stop Asthma crises in the UK
A pragmatic cluster randomised trial with nested economic and process evaluations examining the effects of integrating at-risk asthma registers into primary care with internet-based training and support
Chief Investigator: Prof Andrew Wilson, University of East Anglia
Click here to learn more
The impact of acute systemic inflammation upon cerebrospinal fluid and blood biomarkers of brain inflammation and injury in dementia: A study in acute hip fracture and memory clinic patients
Chief Investigator: Prof Chris Fox, University of East Anglia
A randomised controlled trial of a complex intervention to prevent return to smoking postpartum
Chief Investigator: Prof Caitlin Notley, University of East Anglia
Click here to learn more
Scientific Title:
Biomarkers for Rational Investigation for Neurological decision Support in traumatic brain injury (BRaINS-TBI): cohort study with a nested pragmatic randomised trial
Chief Investigator:
Dr Virginia Newcombe, University of Cambridge and Cambridge University Hospitals NHS Foundation Trust
Click here to learn more
Adapting and testing an intervention for carers of people with dementia
Click here to learn more
Development of a System to Provide an Automatic Diagnosis for Vestibular Conditions
CAVA Clinical Investigation Plan v3.1 25.01.24
CAVA Participant Information Sheet v4.0
Click here to learn more
CompreHensive geriAtRician-led MEdication Review
Chief Investigators: Professor Debi Bhattacharya, University of Leicester and Professor David Wright, University of Leicester
Click here to learn more
Cessation of Smoking Trial in the Emergency Department
Chief Investigators: Dr Ian Pope and Prof Caitlin Notley, University of East Anglia
Click here to learn more
Cognitive Behavioural Therapy for the treatment of post-traumatic stress disorder (PTSD) in youth exposed to multiple traumatic stressors: a phase II randomised controlled trial.
Chief Investigator: Dr Richard Meiser-Stedman, University of East Anglia
A multi-centre, randomised, double-blind, placebo-controlled trial to establish the effect(s) of administration of sertraline (50 mg, once daily for 6 months) in people with a recent stroke and post-stroke emotionalism.
Evaluating Antidepressants for emotionaliSm after strokE (EASE)
Lead investigator: Prof Niall Broomfield, University of East Anglia
Click here to learn more
Developing, optimising and evaluating a conversion of standard smoking cessation support in pregnancy into a digital support package (eSupport)
Norwich CTU, as co-applicants, are providing operational oversight, QA, data management and statistical support.
RCT of group CBT for men with intellectual and/or developmental disabilities and harmful sexual behavior
A feasibility study to assess the design of a multi-centre randomised controlled trial of the clinical and cost-effectiveness of a caregiving intervention for people following hip fracture surgery.
Chief Investigator: Prof Toby Smith, University of East Anglia
Click here to learn more
Norwich CTU are providing trial management, operational oversight and data management for the study.
The link to the study website is below
https://iact4carers.com/
Individualised assessment and management for brain injury app: widening access to
resources for recovery (I am brain aware)
Norwich CTU are providing operational support, data management , statistics and process evaluation .
Interpersonal Counselling for Adolescent Depression delivered by Youth Mental Health Workers without Core Professional Training: A Feasibility Randomised Controlled Trial
Identification of Medication Adherence Barriers Questionnaire intervention (IMAB-Qi)
IMAB-Qi is a five-year England-wide research programme that aims to help patients take their medicines as prescribed.
Co - Chief Investigators: Prof Debi Bhattacharya and Dr Sion Scott, UEA School of Health Sciences
Click here to learn more.
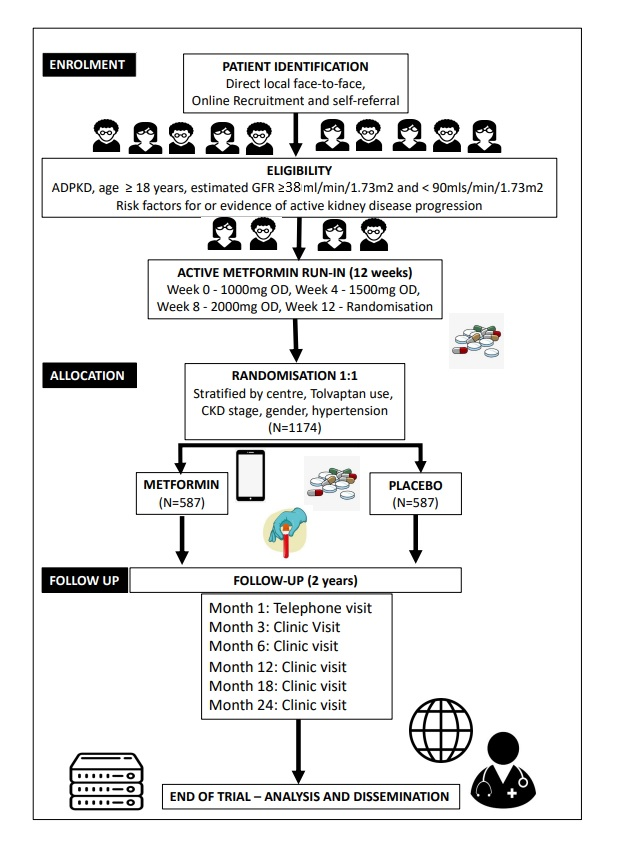
Implementation of Metformin theraPy to Ease Decline of kidney function in Polycystic Kidney Disease : A Randomised Placebo-Controlled Trial
Norwich CTU, as co-investigators, are providing full operational support and oversight, QA, data management and statistical support.
For more on the award click on the link below.
https://fundingawards.nihr.ac.uk/award/NIHR156614
Development, evaluation and implementation of molecular diagnostics for hospital-acquired and ventilator-associated pneumonia in diverse UK hospital settings
Chief Investigators: Dr Vanya Gant, University College London and Prof David Livermore, University of East Anglia
Click here to learn more
Click here to learn more
A feasibility study to assess the design of a multi-centre randomised controlled trial of the clinical and cost-effectiveness of a caregiving intervention for people with chronic musculoskeletal pain
Click here to learn more
Optimising the delivery of Mental health support to adolescents in care via low-intensity life story work: a realist evaluation with co-design and feasibility trial
As co-applicants, Norwich CTU are providing operational support, QA, data management, health economics, Process Evaluation and statistics.
Risk Factors for the Development of Bilateral Ménière's Disease and the Establishment of a National Ménière's Disease Registry
Chief Investigator: Dr John Phillips, Norfolk & Norwich University Hospital
Practical management of cognitive and neuropsychiatric symptoms in MND
Click here to learn more
A Randomised, Double-Blind, Placebo-Controlled, Crossover Design, Phase Iib Clinical Trial to Evaluate the Efficacy and Safety of Oral Atomoxetine for the Treatment of Cognitive and Behavioural Change in Participants with Progressive Supranuclear Palsy Syndromes.
Chief Investigator: Professor James B. Rowe, University of Cambridge
Click here to learn more
Evaluating Palin Stammering Therapy for School Children versus Treatment as Usual: a feasibility trial
Click here to learn more
Click here to learn more
Supportive exercise programmes for accelerating recovery after major abdominal cancer surgery
Chief Investigators: Mr James Hernon, Norfolk & Norwich University Hospital and Professor John Saxton, Northumbria University
Click here to learn more
PRegnancy Outcomes using continuous glucose monitoring TEChnology in pregnant women with Type 2 diabetes: A multicentre randomised controlled trial of the clinical and cost-effectiveness of using continuous glucose monitoring in pregnant women with type 2 diabetes
As co-applicants, Norwich CTU are providing full operational support, QA, data management and statistics.
The efficacy and mechanisms of action of n-3 poly-unsaturated fatty acid supplementation in people with non-steroidal exacerbated airways disease and uncontrolled asthma
Chief Investigator: Prof Andrew Wilson, University of East Anglia
Click here to learn more
Feasibility randomised controlled trial of a smoking cessation smartphone app that delivers ‘context aware’ behavioural support in real time
Chief Investigator: Dr Felix Naughton, University of East Anglia
A Randomised controlled trial of Energetic Activity for Depression in Young people
Chief Investigator: Dr Daksha Trivedi
Click here to learn more
Improving the wellbeing of Children in Care through a group intervention for foster carers: a randomised controlled trial
Chief Investigator: Prof Nick Midgley, University College London
Click here to learn more
To determine the efficacy and safety of faecal microbiome transplantation for ME/CFS
Lead Investigator: Prof Simon Carding, Quadram Institute
Click here to learn more
Research on Efficacy of Teriparatide Use in the Return of recruits
to Normal duty
Click here to learn more
Study of BROdalumab in Primary Sclerosing Cholangitis
Norwich CTU are supporting the fellowship with operational oversight and support, QA, data management and statistical oversight.
Social Prescribing for people to live enjoyably with dementia/memory problems in daily life
As co-investigators, Norwich CTU are providing full operational support, QA, programme management, data management, qualitative support and statistics.
Screening LOg Guidelines (SLOG): A standardised model for screening data
Click here to learn more
Treating people with Idiopathic Pulmonary fibrosis with the Addition of Lansoprazole
Chief Investigator: Prof Andrew Wilson, University of East Anglia
Click here to learn more
Tailoring evidence-based psychological therapY for People with common mental disorder including Psychotic EXperiences
Chief Investigator: Prof Jesus Perez, University of Cambridge
Click here to learn more
Co-design and evaluation of an intervention to increase UPTake of pUlmonary RehabilitatioN for people living with chronic obstructive pulmonary disease: the UPTURN study.
As co-investigators, Norwich CTU are providing overall programme management, QA, operational support, data management and statistics.
Co-designing and Evaluating An Online Self-Help Brief Psychosocial Intervention (eBPI) For Young People With Mood Related Mental Health Problems To Reduce Waiting Lists in Specialist CYPMHS
As co-investigators , Norwich CTU are providing operational support and oversight, QA and data management.
Read more about some of our previous research projects:
Automated Insulin Delivery in Women with Pregnancy Complicated by Type 1 Diabetes
Norwich CTU worked with the CI and TMG to deliver the AiDAPT trial taking on full responsibility for operational delivery of the trial.
The trial was published in the New England Journal of Medicine and contributed to the change in NICE Guidelines for pregnant women living with Type 1 diabetes.
The Efficacy and Mechanism Evaluation of Treating Idiopathic Pulmonary fibrosis with the Addition of Co-trimoxazole
Norwich CTU worked with the CI and TMG to deliver the EME-TIPAC trial taking on full responsibility for operational delivery, data management, QA and statistics for the trial.
The results were published in JAMA
A randomised feasibility study of serial magnetic resonance imaging to reduce treatment times in Charcot neuroarthropathy in people with diabetes
Click here to read the protocol paper
Comparison of the JOURNEY II Bi-Cruciate Stabilised and GENESIS II Total Knee Arthroplasty in Performance and functional Ability
Chief Investigators: Professor Iain McNamara, Norfolk and Norwich University Hospital, and Dr Celia Clarke, University of East Anglia
Click here to read the protocol paper
Click here to read the publication paper
The Care Homes Independent Prescriber Pharmacist Study
Chief Investigator: Prof David Wright, University of East Anglia
Click here to learn more
A multi-centre cluster randomised controlled trial to evaluate the Guide to Action Care Home (GtACH) fall prevention programme in old age UK care homes
Chief Investigator: Prof Pip Logan, University of Nottingham
Click here to learn more
Click here to read the results
Study of Mirtazapine or carBamazepine for Agitation in Dementia
Chief Investigator: Prof Sube Banerjee, Brighton and Sussex Medical School
Click here to read the results
Our other projects
DLPT: Data Linkage and Pseudonymisation tool
Click here to learn more
With funding from the National Institute for Health Research (NIHR), we provided training to assist the adoption of eConsent via REDCap. Between 14th and 28th February 2022, we delivered two webinars and one online workshop and after that, we developed a guidance document.
Click here to learn more
