SLoG

Screening LOg Guidelines (SLOG): A standardised model for screening data: who should be included and which data should be collected?
Norwich CTU received funding in 2021 from the National Institute for Health Research (NIHR) CTU Support Funding scheme to review current practice and develop guidelines on the use of Screening Logs. The views expressed here are our own.
The reporting of trials in health or social care research often includes the number of participants assessed for inclusion into the trial. Such information is traditionally referred to as “screening data”.
Some research had been undertaken by other teams however we were unable to find any national or international guidelines on collecting screening data. Without standardisation or uniform guidance there is lack of clarity and inconsistency around the collection of screening data, opportunities are lost to understand recruitment and reporting bias and at worst the published data may be misleading.
In some trials it may be possible to obtain more complete and representative data on potential participants from registries and / or NHS databases. In these trials the burden of recording trial screening data could be removed from trial sites.
Summary of the SLoG project:
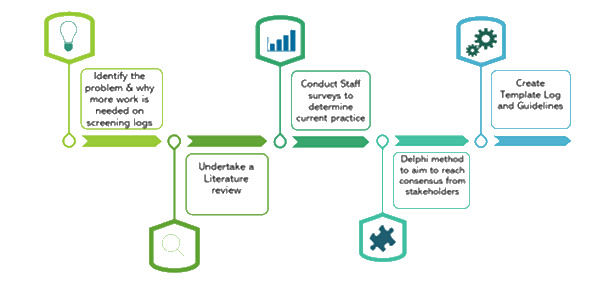
Literature review
We reviewed reporting of screening data in a selection of trials published between 2011 and 2020. The first 91 fulfilling our selection criteria were reviewed, these comprised 44 (48.4%) UK only trials, the remainder were non-UK or trials which included UK sites. The majority 81 (89%) included a consort diagram, or reference to one, but crucially only 36 (40%) included a precise definition of the top line number on the CONSORT diagram “assessed for eligibility” and only 3% mentioned screening logs.
Surveys
We designed two surveys to better understand current use and opinion of screening logs, processes and data. Survey 1 was sent to staff with experience of screening logs, through their work in managing, analysing or supporting recruitment in clinical trials. Survey 2 was sent to clinical staff, those who typically collect screening data.
Survey 1 achieved 113 responses, up to 20th April 2022. Of the respondents,80% were affiliated with a UKCRC registered Clinical Trials Unit; the remainder were based in university/public sector trials units (10%), NHS research departments (5%), non-registered academic CTU (4%) & industry/CRO (1%). Three quarters of respondents had a role in Trial Management (or similar), a further 10% identified as statisticians or data analysts; the remainder reported roles in trial support (4%), data management (4%), QA monitoring (4%) or trial leader/director (4%). The majority of respondents felt that screening logs provide useful information on trials (88%), but that current use of screening logs could be improved (84%).
Survey 2 resulted in 37 responses from nurses (51%), NHS office-based staff (19%), other clinical research roles (16%), doctors (11%) & other HCPs (3%). The majority of respondents (85%) reported that screening data were requested in at least 50% of trials they worked on. Two thirds (68%) used screening logs but felt that the process could be improved.
Delphi Survey
A screening log template with user guidelines was defined and refined using Delphi Survey Rounds.Delphi participants comprised a broad range of stakeholders and we ensured that no roles or job types were over-represented. Participants expressed an interest following completion of either of the surveys detailed and included those who contacted the SLoG team via our social media requests and articles in national trial group newsletters, websites, emails and forums, such as UKCRC, UKTMN, NHS R&D Forum, TMRP and PPI groups as well as our presentation of the SLoG project at the 2022 ICTMC conference in Harrogate.
Findings have been presented at:
6th International Clinical Trials Methodology Conference, Harrogate, UK, 3 – 6th October 2022: ICTMC 2022: 6th International Clinical Trials Methodology Conference -- book of abstracts | Zenodo
44th Annual Meeting of the Society for Clinical Trials, Baltimore, MD, USA, 21st – 24th May 2023
Documents and publications
The initial version of the SLoG guidelines are available to download below.
A template screening in MS Word is available here
The results from the surveys, literature review and Delphi Rounds will be published in an open access journal, a link will be provided on this page when available.
Contact us
Please contact us if you would more information, or to work with the team on future screening log work or to request access to the data (not available until after primary publication).
